Asymmetric catalysis is an advanced area of chemical synthesis, but the handling of abundantly available, purely aliphatic hydrocarbons has proven to be challenging. Typically, heteroatoms or aromatic substructures are required in the substrates and reagents to facilitate an efficient interaction with the chiral catalyst. Confined acids have recently been introduced as tools for homogenous asymmetric catalysis, specifically to enable the processing of small unbiased substrates1. However, asymmetric reactions in which both substrate and product are purely aliphatic hydrocarbons have not previously been catalysed by such super strong and confined acids. We describe here an imidodiphosphorimidate-catalysed asymmetric Wagner–Meerwein shift of aliphatic alkenyl cycloalkanes to cycloalkenes with excellent regio- and enantioselectivity. Despite their long history and high relevance for chemical synthesis and biosynthesis, Wagner–Meerwein reactions utilizing purely aliphatic hydrocarbons, such as those originally reported by Wagner and Meerwein, had previously eluded asymmetric catalysis.
Since Bredig and Fiske discovered a nonenzymatic, modestly enantioselective cinchona alkaloid-catalysedcyanohydrin synthesis in 19123, asymmetric chemical catalysis has evolved extensively and currentlyencompasses reactions with transition metals+5, enzymesz8 and organocatalysts?10.!, However, thecatalytic and enantioselective processing of purely aliphatic hydrocarbons is still extremely challenging.Typical substrates and reagents used in asymmetric catalysis feature heteroatoms or aromatic groups,which enhance reactivity and enable enantiodifferentiation by providing functional groups that engage inspecific interactions with the catalyst. Indeed, to the best of our knowledge, Pfaltz's landmark iridium-catalysed asymmetric hydrogenation of a purely alkyl-substituted olefin stands out as the only example inwhich an only-aliphatic hydrocarbon is catalytically processed to give an enantiopure only-aliphatichydrocarbon!213.14, We became interested in advancing hydrocarbon chemistry by investigating cationicshifts as a fundamentalclass of chemical and biochemical transformations.The most prominent example inthis regard is the cationic Wagner-Meerwein rearrangement1516, Catalytic enantioselective Wagner-Meerwein reactions that proceed through purely aliphatic hydrocarbon-based cations, such as thoseoriginally reported by Wagner and Meerwein, have not previously been described (Fig.la,b !Z18 192021,Notable examples of enantioselective cationic shifts were reported by Trost and Yasukataz2Irost and Xie23 and lacobsen and coworkers24.25, and feature t-allyl palladium intermediates to affordenantioenriched a-vinyl cycloketones (Fig. 1C) or a hypervalent iodine-catalysed, 1,3-difluorinative reactionofB-substituted styrenes (Fig. 1d).
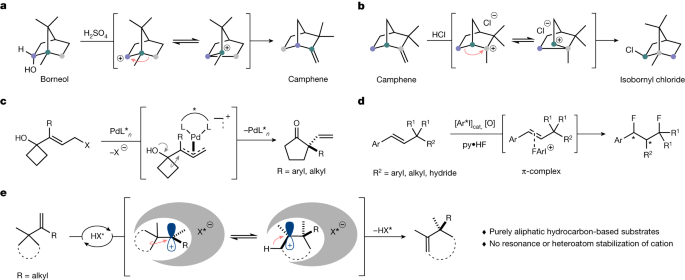
a, Borneol to camphene rearrangement (Wagner 1899)46. b, Camphene to isobornyl chloride rearrangement (Meerwein 1922)47,48. c, Palladium-catalysed semipinacol rearrangement/Wagner–Meerwein shift. d, Aryl iodide-catalysed enantioselective 1,3-difluorination. e, Catalytic asymmetric Wagner–Meerwein shifts of aliphatic hydrocarbons (this work). HX*, Brønsted acid.